Dionise Ysabelle Bawal, MD
To describe the landscape of diabetes management for the past fifteen or so years as exciting would be an understatement.
Before insulin, patients with diabetes had to starve themselves to achieve better glucose control. It came to a point where they were asked to fast for ten days to clear glucosuria. Sadly, these patients died of the complications of diabetes. Optimal glycemic control became a reality when insulin came along, and the torture of starving oneself to death became obsolete. The earliest oral antidiabetic medications were sulfonylureas (SU) and metformin. Up to the early 1990s, diabetes regimen would be a mix and match of human insulin, SU and metformin. Eventually, insulin analogs (i.e., aspart, lispro, glargine) and other oral medications were added to the armamentarium of diabetes, such as thiazolidinediones, alpha-glucosidase inhibitors and meglitinides. Overall, diabetes management was doing fine… Until incretin-based therapy entered the scene and shook things up.
As a child, we were taught that if something sounds too good to be true, it probably is. However, is this also the case with Tirzepatide? Spend the next 10 to 15 minutes with me, and let us find out.
The 401 on Incretins
Remember the incretin effect? A phenomenon whereby administration of oral glucose resulted in greater insulin secretion than intravenous glucose. There are two well-known incretin hormones: gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). GLP-1 is produced in L cells in the distal intestine, while GIP is synthesized within K cells in the duodenum. These incretins rapidly increase with meals or glucose intake, stimulating insulin secretion and ensuring normal glucose tolerance.
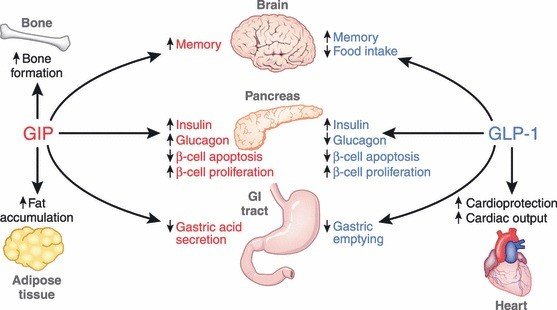
Figure 1. Pancreatic and exopancreatic function of glucose‐dependent insulinotropic polypepide (GIP) and glucagon‐like peptide (GLP)‐1. GIP acts directly on the endocrine pancreas, bone, fat, gastrointestinal (GI) tract and brain.
GIP and GLP1 – are they identical? Not really. GIP was the first incretin discovered. It increases both insulin and glucagon secretion, while GLP1 increases insulin but decreases glucagon secretion. Both incretins increase beta cell proliferation and decrease beta cell apoptosis, leading to an overall increase in beta cell mass. GLP1 suppresses appetite and decreases gastric emptying time. These incretins are degraded by dipeptidyl peptidase-4 (DPP-4); hence, DPP-4 inhibitors, such as sitagliptin or linagliptin, are also under incretin-based therapies.
Returning to the incretin effect, data has shown diminished insulin response to both incretins in patients with type 2 diabetes. This may explain the postprandial glucose elevations and poor overall glycemic control in diabetes. Furthermore, a study showed that GIP infusion caused an increase in blood glucose levels, probably because of the increase in glucagon. Given these seemingly negative findings, can the combination of GLP1/GIP be helpful in diabetes management?
Incretin Magic in Type 2 Diabetes
The paradigm shift in the management of diabetes paved the way for the appreciation of incretin-based therapies. We have shifted from a purely glucocentric approach to a beta cell-centric approach, emphasizing the importance of beta cell preservation and treatment durability. Even more so, treatment selection now includes improvements in cardiorenal and weight-related outcomes.
As aforementioned, the reduced insulin response to incretins in patients with diabetes may theoretically pose a barrier to the effectiveness of incretin-based therapies. However, studies have shown that giving supraphysiological doses of GLP1 (but not GIP) restored the insulin response, allowing better control of both fasting and postprandial glycemic excursions. There is also substantial weight loss from this medication. Exogenous GIP, on the other hand, did not significantly reduce plasma glucose levels among patients with diabetes, lowering its therapeutic potential. Naturally, there was a decline in interest in GIP as an antidiabetic medication and why interest was focused on GLP1. This led to the development of GLP1 receptor agonists (GLP1RA), which have all gained attention and overwhelming responses from both physicians and patients. In addition to solid evidence regarding glycemic and weight control efficacy, GLP1RA also provides direct cardiorenal protection.
Tirzepatide: Double Trouble for Diabetes?
Despite the discouraging evidence for the utility of GIP in diabetes management, there was regained interest in the incretin with the release of Tirzepatide. It is a once-weekly subcutaneous injectable dual GLP1 and GIP receptor agonist. Was combining GLP1 and GIP a good idea? Let us go through the data, shall we?
Getting to know the Twincretin
Tirzepatide is a long-acting GIP/GLP1RA with a half-life of 5 days, allowing for its once-weekly dosing. It is currently approved for the treatment of patients with type 2 diabetes.
Glycemic Control
There is no question regarding the efficacy of GLP1 receptor agonists in achieving good glycemic control. The question is whether adding the GIP was necessary. As we have discussed earlier, exogenous GIP infusion does not cause a significant improvement in insulin secretion in patients with diabetes and can produce transient hyperglycemia.
The SURPASS-2 trial (A Study of Tirzepatide versus Semaglutide Once Weekly as an Add-On Therapy to Metformin in Participants with Type 2 Diabetes) compared the efficacy of different doses of tirzepatide with semaglutide 1 mg in patients with poorly controlled diabetes on metformin monotherapy. After 40 weeks, reduction of glycated hemoglobin (HbA1c) of -2.01, -2.24, -2.30 was seen in the 5 mg, 10 mg, and 15 mg tirzepatide respectively, compared to -1.86 reduction with 1 mg semaglutide. Approximately 86% of patients who received tirzepatide achieved an HbA1c level of less than 7.0%. There was also a greater reduction in fasting blood glucose and 2-hour postprandial glucose levels in the tirzepatide group.
In a network meta-analysis done by Karaginannis, T et al. (2023), which included 22 randomized controlled trials (N= 18,742 T2DM patients), results showed that Tirzepatide brought about more significant reductions in HbA1c from baseline compared to semaglutide, basal insulin and other GLP-1 receptor agonists (Figure 2a). Even compared to the highest approved dose of semaglutide of 2 mg, tirzepatide still proved to be more efficacious in glycemic control.
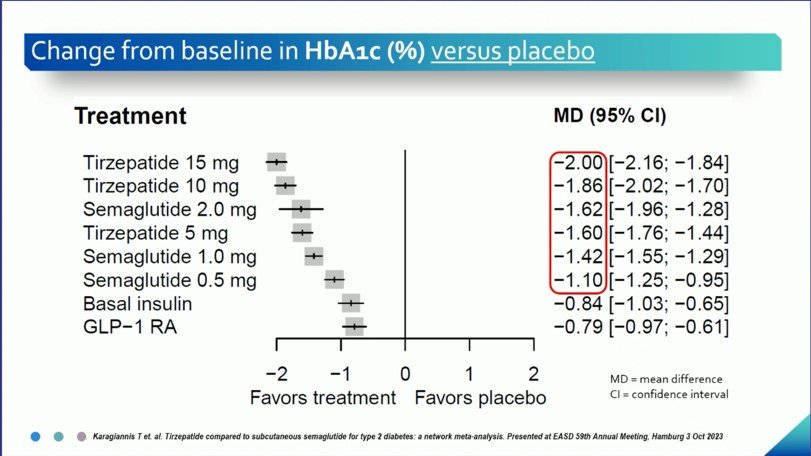
Figure 2a. HbA1c Reduction with Tirzepatide vs other anti-diabetic medications (as presented in EASD 2023)
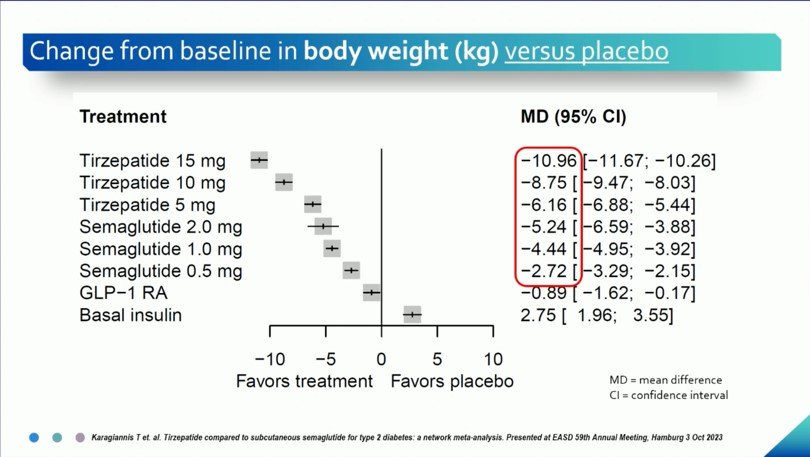
Figure 2b. Weight Reduction with Tirzepatide vs other anti-diabetic medications (as presented in EASD 2023)
One may wonder how a dual GIP/GLP1 receptor agonist works when one increases glucagon and another decreases glucagon, which may seem contradictory. The inhibitory effect of GLP1 on glucagon secretion surpasses the glucagon-inducing effect of GIP, hence leading to effective glucose lowering.
Body weight
Reduction in body weight has been one of the most appealing effects of GLP1RA. Among the other GLP1RA, semaglutide has superior evidence in terms of weight loss, with an average weight loss of 4.5 to 6.5 kg, as seen in the SUSTAIN trials at a dose of 1 mg.
Current data shows that the dual GIP/GLP1 receptor agonist tirzepatide provides even more significant weight reduction than semaglutide. In the SURPASS-2 trial, reductions in body weight appeared to be dose-dependent. At 40 weeks, there was a mean weight reduction of -7.6 kg, -9.3 kg, and -11.2 kg at the 5 mg, 10 mg and 15 mg dose, respectively, while a mean reduction of -5.7 kg with semaglutide 1 mg. Interestingly, even at the lowest dose of tirzepatide, weight loss was superior to semaglutide. In another study known as the SURMOUNT-2 trial, there was a mean weight loss of 12.8% and 14.7% with the 10 and 15 mg tirzepatide, respectively, after 72 weeks of treatment.
In the same meta-analysis done by Karaginannis, T. et al. (2023), tirzepatide was found to have greater reductions in body weight even compared to the highest dose of semaglutide of 2 mg, similar to the findings on glycemic control (Figure 2b).
Obesity
In a survey done in 2022, approximately 38.6 percent of Filipino adults aged 20 to 59 were overweight or obese. This statistic is clinically relevant as obesity increases cardiovascular risk, especially in this relatively young age group. Numerous anti-obesity medications have been prescribed to patients in an attempt to help them lose weight. However, the response to such medications varies for each patient. The effectiveness of GLP1RA for weight loss has been established in research and even in real-world practice. Although only one GLP1RA has been approved by the FDA in the Philippines for weight loss alone (without diabetes), patients have been clamoring to acquire these medications to hasten weight reduction. The first incretin-based therapy to be approved solely for overweight or obesity was the GLP1RA liraglutide. In the Philippines, this is the only one approved for weight loss without diabetes.
Until recently, GIP has not been utilized for weight loss as it is obesogenic. However, when combined with GLP1RA, GIP enhances GLP1R activity, which may explain superior weight loss compared to GLP1RA alone. Another explanation was the presence of neuronal GIP and GLP1 receptors on neuronal tissues. Exogenous GLP1 infusion decreased energy intake, decreased appetite, and decreased brain responses to food anticipation. Therefore, reduced food intake primarily drives weight loss secondary to GLP1RA.
In the SURPASS trials, all participants had type 2 diabetes. One phase III trial was published in 2022 regarding the use of tirzepatide on overweight and obese patients without diabetes, known as the SURMOUNT trial. Results showed that tirzepatide led to a weight loss of 11.9%, 16.4% and 17.8% with a corresponding dose of 5 mg, 10 mg and 15 mg, respectively. There have yet to be studies directly comparing tirzepatide vs semaglutide in this subgroup of patients.
We can only theorize regarding the synergistic effect of GIP and GLP-1 effect on appetite and body weight when combined in one drug. However, its actual mechanism is yet to be elucidated.
Effects on Muscle and Liver
There are extrapancreatic effects that illustrate the myriad of metabolic benefits that tirzepatide can offer. The exact mechanism of these changes is still being investigated.
In the SURPASS-3 MRI, a substudy of the SURPASS-3, the effect of tirzepatide on liver fat content and abdominal adipose tissue in patients with type 2 diabetes (BMI >25 kg/m2) were examined. These patients were pre-screened to have fatty liver disease. Results showed a more significant reduction in liver fat composition (LFC) in the tirzepatide 10 and 15 mg group versus the insulin degludec group. The change in LFC correlated with reductions in abdominal adipose tissue and weight loss.
Loss of muscle mass is an inevitable change that occurs with aging and may occur with weight loss. These changes are said to be worse in individuals with type 2 diabetes. In an exploratory posthoc analysis of the SURPASS-3 MRI substudy, results showed that patients treated with tirzepatide on top of metformin with/without an SGLT-2 inhibitor had a greater than predicted reduction of muscle fat. This study showed that despite significant weight loss and a decrease in fat level, there were no apparent adverse effects on muscle composition in patients treated with tirzepatide.
Safety
The most common adverse effects associated with the use of GLP1RA are gastrointestinal in nature – nausea, vomiting, diarrhea, and anorexia. In the meta-analysis by Karaginannis, T. et al. (2023), there was no significant difference in nausea and diarrhea occurrence between tirzepatide and semaglutide or other GLP1RA. However, in terms of vomiting, results showed higher odds of vomiting in high-dose tirzepatide versus semaglutide 0.5 and 1 mg. There was also no significant difference in treatment discontinuation due to gastrointestinal and other adverse events between the two groups.
Hypoglycemia is an essential aspect of safety in diabetes management. Despite superiority over insulin degludec regarding HbA1c lowering, there are significantly lower episodes of hypoglycemia with tirzepatide. Again, this is secondary to the glucose-dependent mechanism of incretin-based therapies wherein increased insulinotropic activity occurs only in the presence of hyperglycemia.
Tirzepatide and the Heart
The cardiovascular safety and protection offered by GLP1RA have already been established in numerous cardiovascular outcome trials such as SUSTAIN-6, LEADER and REWIND. GLP1RA provide cardiovascular benefit via direct effects on the heart and vasculature, combined with anti-inflammatory and antioxidant effects. Theoretically, we expect the same benefit with tirzepatide, which also has a GLP1RA component. The CVOT for tirzepatide (SURPASS-CVOT) began in May 2020 and is still ongoing. Its estimated completion is in the last quarter of 2024. The SURPASS-CVOT will compare the effect of tirzepatide versus dulaglutide on major adverse cardiovascular events in patients with type 2 diabetes and increased cardiovascular risk.
While waiting for the outcome of the SURPASS-CVOT, we look at one study known as the SURMOUNT-1 trial that included obese patients with weight-related complications such as hypertension, dyslipidemia, sleep apnea or cardiovascular diseases. Although the primary outcome was weight loss, this study had cardiometabolic parameters as its secondary outcomes, such as waist circumference, systolic blood pressure, fasting insulin and lipid levels and physical function score on the 36-item Short Form Health Survey (SF-36). Compared with the placebo, patients with prediabetes initially achieved normoglycemia and had physical function scores on SF-36 that were higher in the tirzepatide group. Mean reduction in total body fat mass was 33.9%. There was a decrease in systolic blood pressure, and in the levels of triglycerides, LDL and fasting insulin with tirzepatide.
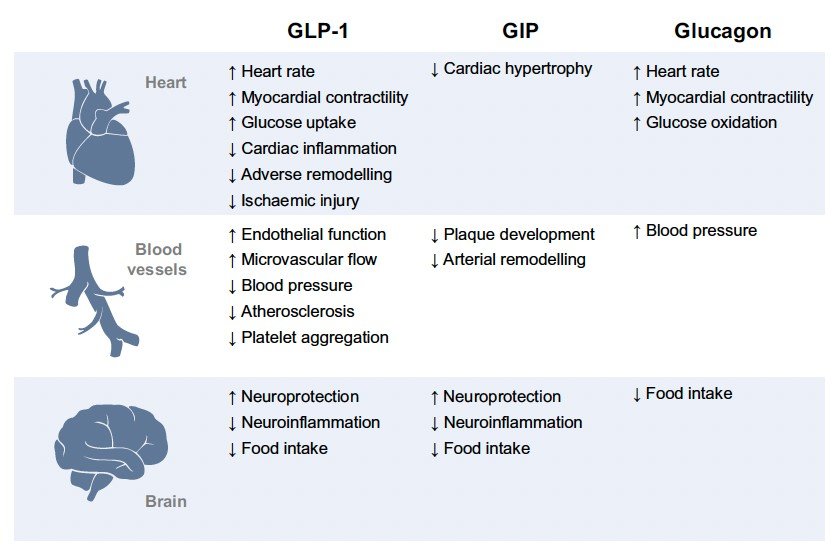
Figure 3. Proposed direct mechanisms of cardiovascular protection exerted by pharmacological activation of GLP-1, GIP and glucagon receptors.
The ability of tirzepatide to address such cardiometabolic risk factors may translate to established cardiovascular benefits in the long run. In mouse models, infusion of GIP was found to have anti-atherogenic effects. A summary of proposed mechanisms for cardiovascular protection with GLP1, GIP and glucagon is illustrated in Figure 3.
Tirzepatide: Two Good to be True?
So, do you think tirzepatide is too good to be true? We have gone through much data to understand the mechanism and benefits of the twincretins. I have always been an optimist, and although some things in life may sound too good to be true, tirzepatide may be the exception.
References:
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23. doi: 10.1111/j.2040-1124.2010.00022.x. PMID: 24843404; PMCID: PMC4020673.
- Frías, J. P., Davies, M. J., Rosenstock, J., Pérez Manghi, F. C., Fernández Landó, L., Bergman, B. K., Liu, B., Cui, X., & Brown, K. (2021). Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine, 385(6), 503–515. https://doi.org/10.1056/nejmoa2107519
- Karagiannis, T et al. (2023). Tirzepatide compared to subcutaneous semaglutide for type 2 diabetes: a network meta-analysis. Presented at EASD 59th Annual Meeting, Hamburg 3 Oct 2023.
- Drucker DJ, Holst JJ. (2023). The expanding incretin universe: from basic biology to clinical translation. Diabetologia special issue. https://doi.org/10.1007/s00125-023-05906-7
- Nauck MA, Müller TD. (2023). Incretin hormones and type 2 diabetes. Diabetologia special issue. https://doi.org/10.1007/s00125-023-05956-x
- Mathieu C, Ahmadzai, I. (2023). Incretins beyond type 2 diabetes. Diabetologia special issue. ttps://doi.org/10.1007/s00125-023-05980-x
- Solini A, Tricò D, Del Prato S. (2023). Incretins and cardiovascular disease: to the heart of type 2 diabetes? Diabetologia special issue. https://doi.org/10.1007/s00125-023-05973-w
- Ludvik, B., Giorgino, F., Jódar, E., Frias, J. P., Fernández Landó, L., Brown, K., Bray, R., & Rodríguez, Á. (2021). Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet (London, England), 398(10300), 583–598. https://doi.org/10.1016/S0140-6736(21)01443-4
- Rayner, C. K., & Horowitz, M. (2021). Twincretin therapy for type 2 diabetes: how do two do?. Lancet (London, England), 398(10300), 560–561. https://doi.org/10.1016/S0140-6736(21)01597-X
- Gastaldelli, A., Cusi, K., Fernández Landó, L., Bray, R., Brouwers, B., & Rodríguez, Á. (2022). Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. The lancet. Diabetes & endocrinology, 10(6), 393–406. https://doi.org/10.1016/S2213-8587(22)00070-5
- Jastreboff AM, Aronne LJ, Ahmad NN et al (2022) Tirzepatide once weekly for the treatment of obesity. N Engl J Med 387:205–216. https://doi.org/10.1056/NEJMoa2206038
- Lilly (2023) Lilly’s tirzepatide achieved up to 15.7% weight loss in adults with obesity or overweight and type 2 diabetes in SURMOUNT-2. Available from: https://investor.lilly.com/node/48776 pdf.
![]()