By: Maria Regina Paz K. Tañada MD
For centuries, prior to the discovery of insulin, diabetes was merely managed with starvation diets, exercise, massage, tobacco, and opium. Patients, especially children, would succumb to their illness in a matter of days or weeks. It was in 1921, when the discovery of insulin championed the treatment for the disease, improving patient survival, and consequently, shifting the disease course from an acute fatal illness to a chronic condition requiring life-long treatment.
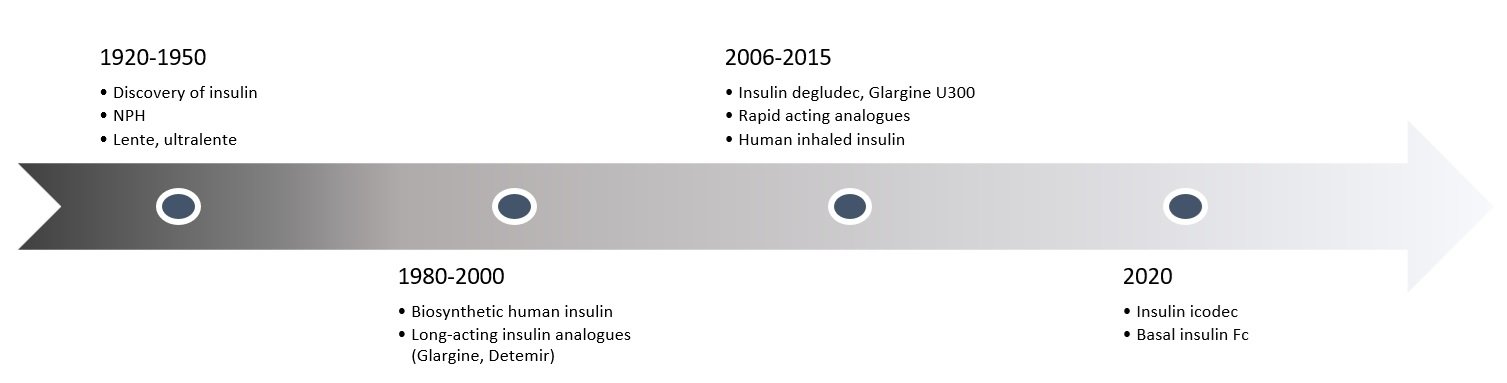
In the past century alone, the ingenuity of science and genetics has evolved insulin production from the initial expensive animal-sourced insulin to synthetic human insulin to insulin analogues. Through genetic engineering, the desired pharmacokinetic and pharmacodynamic properties of insulin were achieved by manipulating the specific sequence of amino acids found in human insulin. This has allowed insulin analogues (e.g. basal and rapid-acting insulin) to appropriately mimic endogenous insulin action. For basal insulin, specifically, the ideal properties include: a longer duration of action, a flat action profile, low day-to-day glycemic variability, and a potential for flexible dosing.
The first generation of basal insulin analogues are glargine U100 and detemir. Both have flatter pharmacokinetic and pharmacodynamic profiles compared to their predecessor (NPH) and have more reliable glucose-lowering activity, with less risk for hypoglycemia. Although glargine U100 and detemir both have a duration of action up to 24 hours, their half-lives are 12 hours and 5-7 hours, respectively.1 This led to the discovery of second-generation basal insulin analogues, degludec and glargine U300, both of which have a flat action profile lasting longer than 24 hours and low day-to-day glycemic variability, allowing for a more consistent once-daily dosing.2,3
As effective as these innovations have been, most patients still do not agree to initiating insulin and, in fact, delay their insulin regimen up to 5 years, even in the presence of diabetes-related complications. 4,5 As for those already prescribed with insulin, more than one-third of patients admit to not strictly adhering to their insulin regimen, in part due to the frequency of daily injections.5 This non-adherence predisposes a patient to have poorer diabetes control, more expenses and a poorer quality of life. Certainly, this has been addressed with increased diabetes education, but what else could be done to encourage insulin adherence?
Enter the ultra-long acting basal insulins.
Following the footsteps of once-weekly GLP1 agonists, ultra long acting insulins were formulated to deliver a weekly dose of basal insulin with a single subcutaneous injection. There have been several of these once-weekly insulins under study, each with varying formulations to slow absorption and to prolong the activity of insulin analogs. This article will be delving into the two ultra long acting insulin that are furthest in the development process: Insulin Icodec and basal insulin Fc (BIF).
| MOLECULE | Company | Phase of development | Diabetes Type | Structural features to enhance activity and prolong half-life |
| Basal Insulin Fc (BIF) | Eli Lilly and Company | Phase 2 | T1D and T2D | Fc-fusion protein |
| Insulin Icodec | Novo Nordisk | Phase 3 | T1D and T2D | Acylation with icosanedioic acid |
Note. Adapted from “Basal weekly insulins: the way of the future!”, by Rosenstock J and Del Prato S, 2022, Metabolism, Volume 126, 154924.
Basal insulin Fc (BIF, or insulin efsitora alfa) is a fusion protein combining a single-chain insulin variant and a human IgG2 Fc domain. Like human insulin, it is internalized by the insulin receptor, but with a lower affinity, resulting in a prolonged plasma half-life of 17 days.6 In patients with type 2 diabetes, BIF exhibited glucose-lowering effects for at least 5 days after a single subcutaneous dose, with the maximum plasma concentration noted on the fourth day.7 There was lower day-to-day and patient-to-patient variability compared to insulin glargine, suggestive of an even flatter action profile that is desired for basal insulin.7
There are three open label treat-to-target phase 2 studies that reported on the safety and efficacy of once-weekly BIF vs once-daily degludec.
The first completed study reported on patients with type 2 diabetes, who were previously treated with basal insulin and oral antidiabetic agents, and were given either insulin degludec or BIF. For patients given BIF, a three-fold loading dose was initially given, then patients were randomized to algorithms targeting FPG of 140mg/dl and 120mg/dl. After 32 weeks, the HbA1c lowering effect of BIF was non-inferior to degludec (-0.58% vs -0.57% vs- 0.66%), and CGM data demonstrated similar glycemic control (TIR 60.5% vs 62.2% vs 63%).8 Hypoglycemia events (<70mg/dl) were 25% lower for BIF compared to degludec, with time below range (<70mg/dl) values as 39% vs 37.1% vs 36.2%.8,9 Remarkably, there was also less weight gain for patients on BIF compared to degludec (+1.0kg vs +1.0kg vs +2.0 kg).
The second phase two study compared BIF vs degludec in insulin naïve patients with type 2 diabetes mellitus. Patients were randomly assigned to once-weekly BIF or once-daily degludec and were titrated to target fasting glucose of 80-100mg/dl. After 26 weeks, HbA1c change was also non-inferior compared to degludec (-1.2% vs -1.26%). CGM parameters demonstrated similar glucose lowering effects with an average TIR of more than 75% by the end of the study period. The rate of hypoglycemia events was low and similar for both groups. There were no severe adverse events or other safety findings reported.10
The third study was conducted on type 1 diabetes patients who received either once-weekly BIF or once-daily degludec. Although HbA1c change was smaller for BIF, the treatment difference of 0.17% (90% CI 0.02-0.32) makes BIF non-inferior compared to degludec. CGM data showed higher fasting glucose values in the BIF group over the 26-week study period, but with similar glycemic variability and hypoglycemia events. There was also a smaller weight gain noted in BIF group compared to degludec (0.1kg vs 0.6kg). 10,11
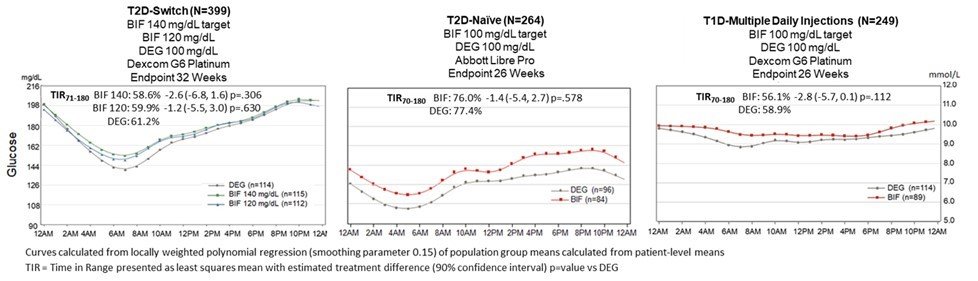
Note. Lifted from “806-P: Once Weekly (QW) Basal Insulin Fc (BIF) and Daily Degludec (DEG) Achieve Comparable Glycemic Control in Three Patient Populations Studied during Phase 2—Clinical Insights from CGM Assessments.” by Kazda C.M., Bue-Valleskey B.M., Zhang Q., Haupt A., Frias J.P. and Dahl D., 2023, Diabetes, 72 (Supplement_1)
The other investigational once-weekly insulin is Icodec. Its ultra-long pharmacokinetic profile is attributed to its strong but reversible albumin-binding, reduced enzymatic degradation and receptor-mediated clearance, achieving a plasma half-life of 196 hours. 12 When insulin icodec is injected, the insulin-albumin forms an inactive depot wherein the insulin is slowly released to target tissues to stimulate glucose lowering. After 3 to 4 subsequent weekly injections, icodec reaches steady state, wherein the maximum-glucose lowering effect is achieved.12
Insulin icodec was well tolerated in the completed phase 2 clinical trials and demonstrated similar glycemic control to insulin glargine among insulin naïve type 2 diabetes patients and type 2 diabetes patients previously treated with basal insulin.13-15 CGM data showed longer TIR (70-180 mg/dl) with less aggressive glucose targets (80-130 mg/dl) and longer TBR (<70mg/dl) with more aggressive glucose targets (70-108 mg/dl) among insulin naïve patients.14 A 100% loading dose of icodec also demonstrated a statistically significant longer TIR compared to insulin glargine group, with attenuation of the initial hyperglycemia seen in the patients who did not receive a loading dose.15 Hypoglycemia rates remained similar, even with the loading dose of insulin icodec. Weight gain was also noted to be similar compared to glargine U100 (range 0.6-1.5kg).13-15
Following the success of the phase II clinical trials, six phase IIIa clinical trials were carried out (ONWARDS program) to evaluate the efficacy and safety of icodec. Among patients with type 2 diabetes who were insulin naïve and on once or twice daily basal insulin, insulin icodec significantly reduced HbA1c compared to once daily insulin glargine or insulin degludec.16-18,20 Continuous glucose monitor (CGM) recorded time in range (TIR) was significantly higher for insulin icodec compared to insulin glargine.16 Combined clinically significant hypoglycemia (<70 mg/dl and <54 mg/dl) was comparable16-18,20, with icodec achieving more target a1c <7% without level 2 or level 3 hypoglycemia.18,20 Increased body weight with icodec was not statistically significant16,18,20, except in one report compared to insulin degludec (1.4kg vs -0.3kg after 26 weeks).17 Among patients with type 2 diabetes on basal-bolus regimen, insulin icodec demonstrated similar HbA1c reduction when used with insulin aspart, compared to insulin glargine u100 with insulin aspart, with no increased risk of hypoglycemia.19 Patients on insulin icodec were reported to have better compliance scores and were more satisfied with their treatment.20
Among patients who have had type 1 diabetes for 19-20 years, insulin icodec (with insulin aspart) demonstrated similar HbA1c reduction compared to insulin degludec (with insulin aspart).21 Combined clinically significant hypoglycemia was significantly higher among patients on insulin icodec (with aspart), with time below range ( <54mg/dl) at the target threshold of 1% during weeks 22-26.21 Interestingly, patient satisfaction scores were significantly lower with insulin icodec compared to degludec, particularly in the areas of flexibility and convenience.21 This suggests that patients with longstanding type 1 diabetes prefer the flexibility of adjusting daily basal insulin dose depending on their daily lifestyles.
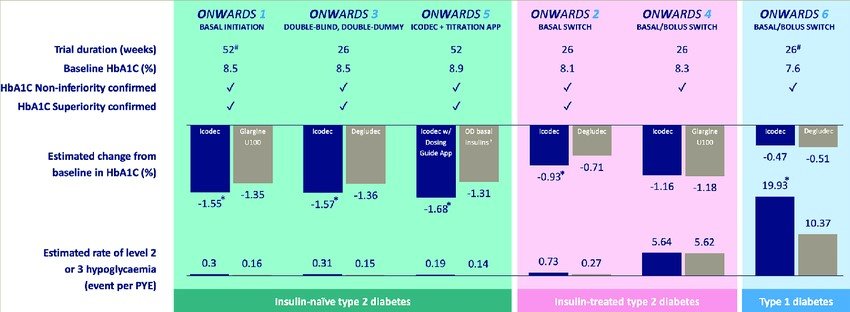
Note. Lifted from “Insulin Icodec Weekly: A Basal Insulin Analogue for Type 2 Diabetes” by Bajaj H and Goldenberg R, 2023, European Endocrinology. 19. 4. 10.17925/EE.2023.19.1.4.
Although both BIF and icodec have demonstrated the potential for excellent glycemic control, with long durations of action and flat action profiles, phase 3 trials are still under development for BIF to further establish its safety and efficacy. Icodec, on the other hand, is expecting a decision from FDA by April 2024.
Once these weekly basal insulins are approved, issues in clinical practice include the standardized initiation dosing and titration algorithms. Transient hyperglycemia in the initial weeks may warrant a higher initial dose of the basal insulin or more stringent short-acting insulins. Clinicians should also be aware of how to titrate weekly doses and monitor for hypoglycemia. Managing type 1 diabetes may be more challenging in terms of dose titration, but should still be considered case to case basis. Although there are no recommendations on patients with critical illness, it might be more prudent to initiate these weekly insulins once out of critical illness since dose titration will be difficult. Concomitant oral diabetes agents should also be maintained for type 2 diabetes patients.
If the past year was any indication, a once-weekly injectable medication will indeed be a more appealing choice for patients with diabetes. Just as the once-weekly GLP-1 agonists were more appealing to patients, decreased injection burden may lead to improved patient compliance and medication adherence. This would then result in decreased rates of complications and fewer hospitalizations for both type 1 and type 2 diabetes patients.
This is a new era. The emergence of 3rd generation basal insulins, the ultra-long acting insulins with the potential for better glycemic control, low risk of hypoglycemia, better patient adherence, lower hospitalizations, and overall, a better quality of life.
REFERENCES:
- I.B. Hirsch, R. Juneja, J.M. Beals, C.J.Antalis, E.E. Wright. The evolution of insulin and how it informs therapy and treatment choices. Endocrinology Review, 41 (2020), pp. 733-755
- Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet. 2014 Sep;53(9):787-800. doi: 10.1007/s40262-014-0165-y.
- R. Ritzel, R. Roussel, A. Giaccari, J.Vora, C. Brulle-Wohlhueter, H. Yki-Järvinen. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab, 20 (2018), pp. 541-548
- Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in insulin therapy study.Diabet Med.2012;29:682–89.
- Rubino, A., McQuay, L.J., Gough, S.C., Kvasz, M. and Tennis, P. (2007), Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with Type 2 diabetes: a population-based analysis in the UK. Diabetic Medicine, 24: 1412-1418.
- HeiseT,Chien J, Beals JM, et al. Pharmacokinetic and pharmacodynamic properties of the novel basal insulin Fc (insulin efsitora alfa), an insulin fusion protein in development for once-weekly dosing for the treatment of patients with diabetes. Diabetes Obes Metab. 2023; 25(4): 1080-1090.
- Tim Heise, Jenny Chien, John Beals, Charles Benson, Oliver Klein, Julie S Moyers, Axel Haupt, Edward J Pratt, Basal Insulin Fc (BIF), A Novel Insulin Suited For Once Weekly Dosing For The Treatment of Patients With Diabetes Mellitus,Journal of the Endocrine Society, Volume 5, Issue Supplement_1, April-May 2021, Page A329,
- Kazda C, Chien J, Zhang Q, Chigutsa E, Landschulz W, Wullenweber O, Haupt A, Frias J. Glycemic Control with Once-Weekly Basal Insulin Fc (BIF) in Persons with Type 2 Diabetes Mellitus (T2DM) Using Continuous Glucose Monitoring (CGM) in a Phase 2 Study.Diabetes1 June 2021; 70 (Supplement_1): 192–OR.
- Juan Frias, Jenny Chien, Qianyi Zhang, Emmanuel Chigutsa, William Landschulz, Kristen Syring, Paula Wullenweber, Axel Haupt, Christof Kazda,Safety and efficacy of once-weekly basal insulin Fc in people with type 2 diabetes previously treated with basal insulin: a multicentre, open-label, randomised, phase 2 study, The Lancet Diabetes & Endocrinology, Volume 11, Issue 3,2023, Pages 158-168,
- Juliana M. Bue-Valleskey,Christof M. Kazda,Chenchen Ma, Jenny Chien, Qianyi Zhang, Emmanuel Chigutsa, William Landschulz, Axel Haupt, Juan P. Frias; Once-Weekly Basal Insulin Fc Demonstrated Similar Glycemic Control to Once-Daily Insulin Degludec in Insulin-Naive Patients With Type 2 Diabetes: A Phase 2 Randomized Control Trial. Diabetes Care 1 May 2023; 46 (5): 1060–1067.
- Christof M. Kazda,Juliana M. Bue-Valleskey,Jenny Chien, Qianyi Zhang, Emmanuel Chigutsa, William Landschulz, Paula Wullenweber, Axel Haupt, Dominik Dahl; Novel Once-Weekly Basal Insulin Fc Achieved Similar Glycemic Control With a Safety Profile Comparable to Insulin Degludec in Patients With Type 1 Diabetes. Diabetes Care 1 May 2023; 46 (5): 1052–1059
- Nishimura E, Kjeldsen T, Hubalek F, Glendorf T, Stidsen C, Hansen B, Pedersen T, Luetzen A, Pridal L, Madesn L. Molecular and Biological Properties of Insulin Icodec, a New Insulin Analog Designed to Give a Long Half-Life Suitable for Once-Weekly Dosing.Diabetes1 June 2020; 69 (Supplement_1): 236–OR.
- Rosenstock J, Bajaj HS, Janež A, et al.; NN1436-4383 Investigators. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med 2020;383:2107–2116
- Harpreet S. Bajaj,Richard M. Bergenstal,Andreas Christoffersen, Melanie J. Davies, Amoolya Gowda, Joakim Isendahl, Ildiko Lingvay, Peter A. Senior, Robert J. Silver, Roberto Trevisan, Julio Rosenstock; Switching to Once-Weekly Insulin Icodec Versus Once-Daily Insulin Glargine U100 in Type 2 Diabetes Inadequately Controlled on Daily Basal Insulin: A Phase 2 Randomized Controlled Trial. Diabetes Care 1 July 2021; 44 (7): 1586–1594.
- Lingvay I, Buse JB, Franek E. et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care 2021;44:1595–1603
- Rosenstock J, Bain SC, Gowda A, Jódar E, Liang B, Lingvay I, Nishida T, Trevisan R, Mosenzon O; ONWARDS 1 Trial Investigators. Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N Engl J Med. 2023 Jul 27;389(4):297-308.
- Philis-Tsimikas A, Asong M, Franek E, Jia T, Rosenstock J, Stachlewska K, Watada H, Kellerer M. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. Epub 2023 May 3. Erratum in: Lancet Diabetes Endocrinol. 2023 Jul;11(7):e9.
- Lingvay I, Asong M, Desouza C, Gourdy P, Kar S, Vianna A, Vilsboll T, Vinther S, Mu Y. Better Glycemic Control with Once-Weekly Insulin Icodec vs. Once-Daily Insulin Degludec in Insulin-Naïve Type 2 Diabetes (ONWARDS 3).Diabetes20 June 2023; 72 (Supplement_1): 178–OR.
- Mathieu, Chantal et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. The Lancet, Volume 401, Issue 10392, 1929 – 1940
- Bajaj HS, Aberle J, Davies M, Donatsky AM, Frederiksen M, Yavuz DG, Gowda A, Lingvay I, Bode B. Once-Weekly Insulin Icodec With Dosing Guide App Versus Once-Daily Basal Insulin Analogues in Insulin-Naive Type 2 Diabetes (ONWARDS 5) : A Randomized Trial. Ann Intern Med. 2023 Nov;176(11):1476-1485. doi: 10.7326/M23-1288. Epub 2023 Sep 26. Erratum in: Ann Intern Med. 2023 Nov 7;: PMID: 37748181.
- Once-weekly insulin icodec versus once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): a phase 3a, randomised, open-label, treat-to-target trial Russell-Jones, David et al. The Lancet, Volume 402, Issue 10413, 1636 – 1647