Yves Jean Y. Liong, MD, FPCP, DPCEDM
Type 1 diabetes is an autoimmune condition that destroys pancreatic β cells, requiring insulin therapy for optimal glycemic control and to prevent complications. While advancements in management have reduced rates of mortality, renal failure, and neuropathy, improvements in nephropathy and proliferative retinopathy remain limited1. Additionally, the risk of hypoglycemia continues to challenge tight glycemic control, impacting social, emotional, and physical well-being for patients and their caregivers2,3.

In recent years, researchers have focused on how immunotherapy might help prevent or slow down the progression of type 1 diabetes. The disease develops in three stages 2:
First Stage: This stage is identified by the presence of two or more autoantibodies, but the individual is still euglycemic.
Second Stage: Here, the individual has both autoantibodies and glucose intolerance.
Third Stage: This stage is marked by noticeable symptoms of hyperglycemia, along with the presence of islet autoantibodies.
Researchers are investigating various immunotherapeutic agents to effectively target each of these stages.
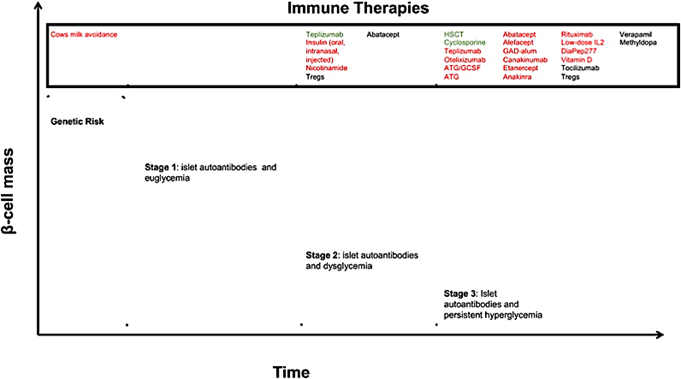
“New Frontiers in the Treatment of Type 1 Diabetes,” by J.T. Warshauer et al, 2020, Cell Metabolism, 31, 46-61.
Cyclosporine, a calcineurin inhibitor, has been shown to improve diabetes remission in patients on insulin for less than two months. However, long-term use carries risks of nephrotoxicity and cancer, prompting the search for safer alternatives. Other agents targeting T-cells, such as Abatacept, and B-cells like Rituximab have been studied, but they have not yet shown significant results 2.
A newer class of immunotherapeutic agents is being explored for managing type 1 diabetes. The first anti-CD3 monoclonal antibody, Muromonab-CD3, was initially approved to prevent organ rejection but had limited use due to adverse cytokine storms. Teplizumab, a humanized version with a better safety profile, was developed to modulate immune cells and protect insulin-producing beta cells, preserving their function4.
Several clinical trials have evaluated Teplizumab for type 1 diabetes, focusing on patients within 12 months of diagnosis undergoing a 12-14 day treatment regimen. Results showed significant preservation of C-peptide levels, indicating maintained insulin production and reduced reliance on exogenous insulin for up to 2 years. Adverse events were generally mild to moderate and self-limited. A cytokine release storm occurred in 5.8% of the Teplizumab group (compared to 1.2% in the control), typically arising within the first 3-5 days and resolving within 2-3 days. Infection rates were similar in both groups5.
Teplizumab is the first drug approved by the US FDA to delay the onset of type 1 diabetes in patients aged 8 and older. According to the latest American Diabetes Association Standards of Care, teplizumab infusions may now be considered for select patients in stage 2 type 1 diabetes to delay the progression to stage 36.
Ongoing clinical trials are further investigating the role of Teplizumab in managing type 1 diabetes, including the potential benefits of a second course and its combination with other immunologic agents. Promising results from recent studies have opened new avenues for preventing or delaying the onset of type 1 diabetes. As research advances and new treatments emerge, the focus may soon shift from merely managing hyperglycemia to preventing or even reversing the disease.
Reference:
- Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ: The 30-Year Natural History of Type 1 Diabetes Complications. Diabetes. 2006, 55:1463–9. 10.2337/db05-1423
- Warshauer JT, Bluestone JA, Anderson MS: New frontiers in the treatment of Type 1 diabetes. Cell Metabolism. 2019, 31:46–61. 10.1016/j.cmet.2019.11.017
- Allen V, Mahieu A, Kasireddy E, Shouman W, Pourrahmat M-M, Collet J-P, Cherkas A: Humanistic burden of pediatric type 1 diabetes on children and informal caregivers: systematic literature reviews. Diabetology & Metabolic Syndrome. 2024, 16:. 10.1186/s13098-024-01310-2
- Thakkar S, Chopra A, Nagendra L, Kalra S, Bhattacharya S: Teplizumab in Type 1 diabetes mellitus: an updated review. touchREVIEWS in Endocrinology. 2023, 19:7. 10.17925/ee.2023.19.2.7
- Herold KC, Gitelman SE, Gottlieb PA, Knecht LA, Raymond R, Ramos EL: Teplizumab: a Disease-Modifying therapy for Type 1 diabetes that preserves Β-Cell function. Diabetes Care. 2023, 46:1848–56. 10.2337/dc23-0675
- ElSayed NA, Aleppo G, Bannuru RR, et al.: 3. Prevention or delay of diabetes and associated comorbidities: Standards of Care in Diabetes—2024. Diabetes Care. 2023, 47:S43–51. 10.2337/dc24-s003